 As CancerCare continues its life-defining work to meet the needs of people facing a cancer diagnosis, we recognize that recent scientific and clinical breakthroughs in cancer treatment present both hopeful progress and practical challenges. This dichotomy is especially acute when it comes to precision oncology: the variability of individual cancers even among tumor types and the availability of increasing numbers of targeted cancer treatments designed to attack specific aspects of an individual’s disease.
As CancerCare continues its life-defining work to meet the needs of people facing a cancer diagnosis, we recognize that recent scientific and clinical breakthroughs in cancer treatment present both hopeful progress and practical challenges. This dichotomy is especially acute when it comes to precision oncology: the variability of individual cancers even among tumor types and the availability of increasing numbers of targeted cancer treatments designed to attack specific aspects of an individual’s disease.
While these treatments offer real hope to people with cancer, they are predicated on timely and comprehensive tests to identify the biomarkers that indicate which treatments are best for them. Unfortunately, many insurance plans do not cover appropriate and comprehensive biomarker testing, which creates a real risk that patients will not receive the most effective therapy, and instead receive sub-optimal treatment, or even treatment that could cause them harm. The human and economic costs of these delays and gaps in care are significant.
As a supplement to CancerCare’s landmark Employer Toolkit focused on best practices for prescription drug benefit design, we are pleased to offer this Employer Toolkit on Biomarker Testing, to help those involved in benefit design make optimal decisions about covering cancer biomarker testing for their employees. Our recommendations reflect best practice policy models that rely on evidence-based guidelines put forth by independent, expert organizations to determine appropriate testing coverage.
Healthcare benefit design is critical to ensuring that cancer patients have timely access to the right care at the right time. We hope you will find this toolkit useful as you develop meaningful and cost-effective coverage policies for your employees.
Sincerely,
Patricia J. Goldsmith
Biomarkers and Biomarker Testing
The oncology treatment landscape is more dynamic and precise than ever, with new scientific and clinical discoveries happening at a breathtaking pace.
 Research continues to demonstrate the positive impact of precision medicine on patient outcomes, which relies on testing to identify how specific biomarkers within a person’s tumor can respond to precisely targeted treatments. Timely and open access to this type of tumor testing enables clinicians to determine the optimal treatment plan for each patient, which may include enrollment in a clinical trial.
Research continues to demonstrate the positive impact of precision medicine on patient outcomes, which relies on testing to identify how specific biomarkers within a person’s tumor can respond to precisely targeted treatments. Timely and open access to this type of tumor testing enables clinicians to determine the optimal treatment plan for each patient, which may include enrollment in a clinical trial.
Biomarker testing has been required or recommended for most of the cancer drugs introduced since 2017. Today there are many kinds of biomarker tests that are proven to help guide treatment decisions for a range of different cancer types, with new versions being rapidly developed. Many experts believe we are quickly moving toward a time when every cancer patient should have biomarker testing at diagnosis and recurrence to help guide treatment decision-making.
Importantly, despite the clear connection between biomarker testing, precision medicine, and improved treatment outcomes, insurers often do not provide adequate coverage. Although many commercial self-insured/large-group employers provide some coverage, policy terms vary since there are currently no consistent minimum coverage requirements for biomarker testing. Consequently, many patients are denied the benefits of these critical diagnostic tools. Furthermore, barriers to testing access can exacerbate health inequities, leading to sub-optimal treatment plans and outcomes among populations already struggling with healthcare disparities.
To maximize opportunities for the best and most equitable care and access to appropriate treatment, CancerCare calls on employers and administrators of self-insured/large group health plans to insist on coverage that ensures their members and beneficiaries have access to timely, guideline-recommended biomarker testing and optimized oncology treatment plans.
This Toolkit is designed to help employers understand the role and benefits of cancer biomarker testing, so they can ask the important relevant questions of their consultants and third-party administrator or healthcare insurance provider, avoid coverage provisions that could negatively impact covered employees, and design coverage that best meets the needs of their employees. A glossary of key terms is included. We've also developed a biomarker testing fact sheet for patients.
Cancer and Mutations
Cancer is a disease in which certain cells grow uncontrollably and can spread to other parts of the body. It is caused by DNA changes to genes that control the way a cell functions, especially how it grows and divides. Biomarkers are specific gene mutations that may indicate important characteristics of the cancer, including how it may grow and spread, its sub-type, and if or how well the patient may respond to a particular treatment. (See glossary for more terms.)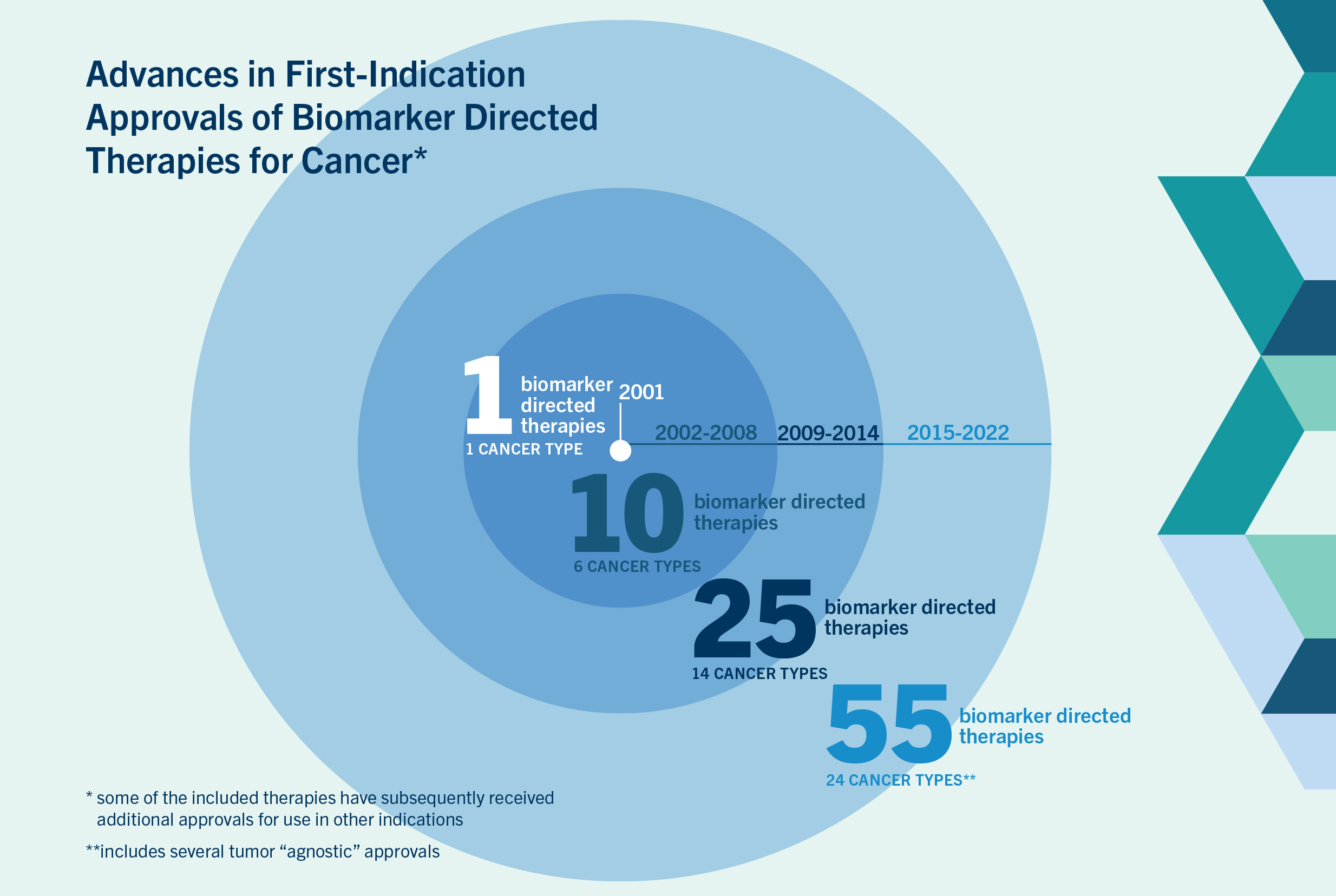
Figure 1 Source
Precision Treatment Through Biomarker Testing
 Precision medicine in oncology has dramatically changed the way cancer is diagnosed and treated thanks to advances like the sequencing of the human genome and more cost effective genome sequencing technologies. Continued technological advances in DNA sequencing tools make them faster and cheaper to use and are leading to much more precise cancer diagnoses and treatments that can effectively target specific molecular sub-types of cancer1.
Precision medicine in oncology has dramatically changed the way cancer is diagnosed and treated thanks to advances like the sequencing of the human genome and more cost effective genome sequencing technologies. Continued technological advances in DNA sequencing tools make them faster and cheaper to use and are leading to much more precise cancer diagnoses and treatments that can effectively target specific molecular sub-types of cancer1.
The list of cancers with clinically valid biomarkers includes many common cancers like lung cancer, breast cancer, melanoma, and colorectal cancer, as well as rare cancers like bile duct cancer2. Fueled by continual advances in research, this list is rapidly growing.
Although cancers are often defined by where in the body they occur, many new treatments effectively target cancers based on specific biomarkers, rather than the tumor’s location. Targeting biomarkers has led to significantly more effective cancer treatment with fewer side effects for patients. Precision cancer treatments include targeted therapies (aimed at specific traits of cancer cells) and biomarker-guided immunotherapy treatments that help a patient’s immune system fight their cancer 1, 2.
Case Study
 Juanita Segura
Juanita Segura
East Chicago, IN
Lung Cancer
Watch Juanita's story »
When the wheezing started in June 2014, Juanita Segura was a 45-year-old mother of five in East Chicago, Indiana. A couple of months later, she developed a cough, and her primary care physician treated her for asthma symptoms. In September, Juanita had a chest x-ray and bloodwork to rule out pneumonia and was started on a course of prednisone.
After two additional months of worsening symptoms, Juanita saw a pulmonologist. Her new doctor noticed an enlarged lung on her x-rays. He gave her a new drug but five days later a very sick Juanita was at the ER, where she had more tests. The stumped doctors finally landed on a possible diagnosis – an infection acquired from her tropical bird – and she was sent off to another specialist. When the bird was found not guilty, Juanita pushed for a CT scan.
The scan showed fluid in her lungs and Juanita was admitted to the hospital. She had a bronchoscopy and two days later was diagnosed with stage 3 malignant lung cancer. Juanita’s husband Steve kicked into high gear, researching treatments and facilities, and took Juanita to Chicago for a second opinion. Steve had read about biomarker testing and the doctors at the cancer treatment center agreed that she should be tested but were unable to get an adequate tissue sample immediately. While they waited, Juanita started chemotherapy and radiation treatments that proved to be ineffective. Six weeks after her initial diagnosis, Juanita learned that her cancer was positive for the ALK biomarker – a mutation for which there are effective targeted therapies. She was prescribed a therapy that targets the ALK mutation, but her employer-based insurance company denied coverage. Juanita appealed and although she won, the start of that treatment was delayed by the insurance company’s prior authorization requirement and her need to appeal the denial.
Juanita’s biomarker testing identified a tumor mutation that was known to be treatable by the targeted therapies that saved her life. Two years after her diagnosis, Juanita Segura opened her own CrossFit Gym, setting an example of fortitude and courage for her clients. “Even if you have a diagnosis, it shouldn’t stop you,” she said. “It’s not going to stop me,” and it hasn’t.
Juanita is now eight years into her cancer experience and taking her 4th targeted treatment. She and Steve are planning a trip to Greece and expecting their second grandchild. Without biomarker testing, Juanita would have suffered through ineffective chemo and radiation and would not have lived to meet even her first grandchild.
Biomarker Testing
Biomarkers are identified through biomarker testing, sometimes referred to as molecular profiling and genome sequencing3.
Multiple types of tests are available to identify the specific characteristics of a patient’s cancer, with the goal of identifying the best treatment4
Some tests check for a single biomarker (single analyte tests), while others look for many biomarkers at the same time (panel test).
Some tests are focused on a certain type of cancer (e.g., lung cancer, breast cancer, or melanoma), while others are focused on individual biomarkers that can be found in multiple types of cancer (e.g.,
BRAF mutations).Next Generation Sequencing (NGS) looks at all the DNA (within and outside of genes) in the cancer.
Some biomarker tests evaluate tumor tissue from a surgical biopsy of the cancer. A new class of biomarker tests, known as liquid biopsies, is designed to evaluate blood or other fluids to identify cancer biomarkers.
Biomarker Testing Landscape
The landscape for biomarker testing in cancer is rapidly evolving as new discoveries are made and new technologies are developed. Once a biomarker has been identified, it is necessary to have a reliable test that can accurately measure it within an individual’s cancer5.*
In the United States, biomarker tests are subject to multiagency regulatory oversight. Those used to select an appropriate therapy are called companion diagnostics. Evidence from clinical trials is used to demonstrate that these tests can identify the biomarker and that the therapy is safe and effective for the patients with that biomarker6. Companion diagnostics require review by the FDA at the same time as a related new drug.
US clinical laboratories develop their own biomarker tests under a different set of rules, overseen by the US Centers for Medicare and Medicaid Services (CMS) and a framework known as CLIA (Clinical Laboratory Improvement Amendments). These types of tests are referred to as Lab-Developed Tests or LDTs. Both types of tests are important to clinicians and patients in making the most appropriate decisions about cancer treatment.
*There are multiple concepts that are evaluated within the healthcare system (especially among researchers, clinicians, regulators, and payors) to determine whether a certain test is reliable for use. These are known as Analytical Validity, Clinical Validity, Clinical Utility.
Biomarker Testing to Guide Cancer Treatment
In today’s oncology clinical practice, timely information about cancer biomarkers is central to treatment planning for a growing number of cancer types and subtypes. A vast majority of oncologists (89%) surveyed in 2021 said biomarker testing makes it easier for them to make more informed treatment recommendations, and 85% agree that improving access to biomarker testing and subsequent treatment informed by biomarker testing is important for advancing health equity7.
Evidence is rapidly mounting that biomarker testing and biomarker-directed therapy improves outcomes for patients8.
“Access to biomarker testing is an important health equity issue. For certain populations, biomarker testing is routine and a game changer for the course of their cancer, while other populations, for a variety of reasons, are not able to equally access this potentially life-saving tool.”
--Jan Kitajewski, PhD
Director, University of Illinois Cancer Center
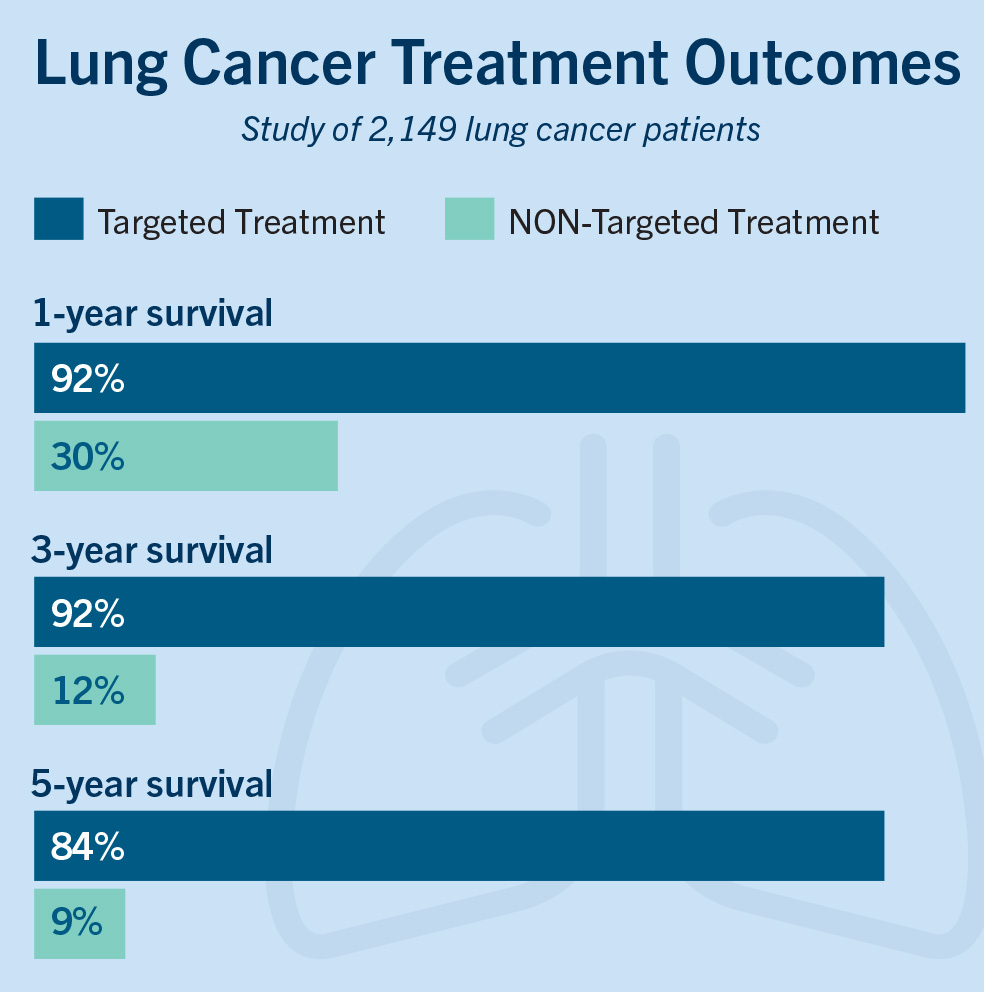 Lung cancer is the leading cause of cancer death worldwide and has been extremely difficult to treat. With the help of biomarker testing, targeted treatment, and immunotherapy, survival rates have soared, providing much encouragement to patients, clinicians, and researchers. A large study of lung cancer patients in Thailand10 shows the impact that targeted treatment can have on survival.
Lung cancer is the leading cause of cancer death worldwide and has been extremely difficult to treat. With the help of biomarker testing, targeted treatment, and immunotherapy, survival rates have soared, providing much encouragement to patients, clinicians, and researchers. A large study of lung cancer patients in Thailand10 shows the impact that targeted treatment can have on survival.
Chart adapted from a comprehensive update from the Srinagarind Hospital-Based Cancer Registry from 2013 to 2017. Asian Pacific Journal of Cancer Prevention, 22(8), 2501–2507.10
Such positive impact from precision oncology is also seen among other cancer types, including melanoma, breast cancer, and colorectal cancer.
- A 2021 study among people with a variety of rare sub-types of cancer found that administering biomarker testing and targeting treatments could improve outcomes for patients with poor prognoses. In this study, patients were given therapies based on their tumor’s profile, before any other (likely ineffective and toxic) generalized therapies were administered, thereby creating efficiencies in treatment, and preventing side effects.11
Case Study
 Rebecca Muñoz
Rebecca Muñoz
East Austin, TX
Breast Cancer
Watch Rebecca's story »
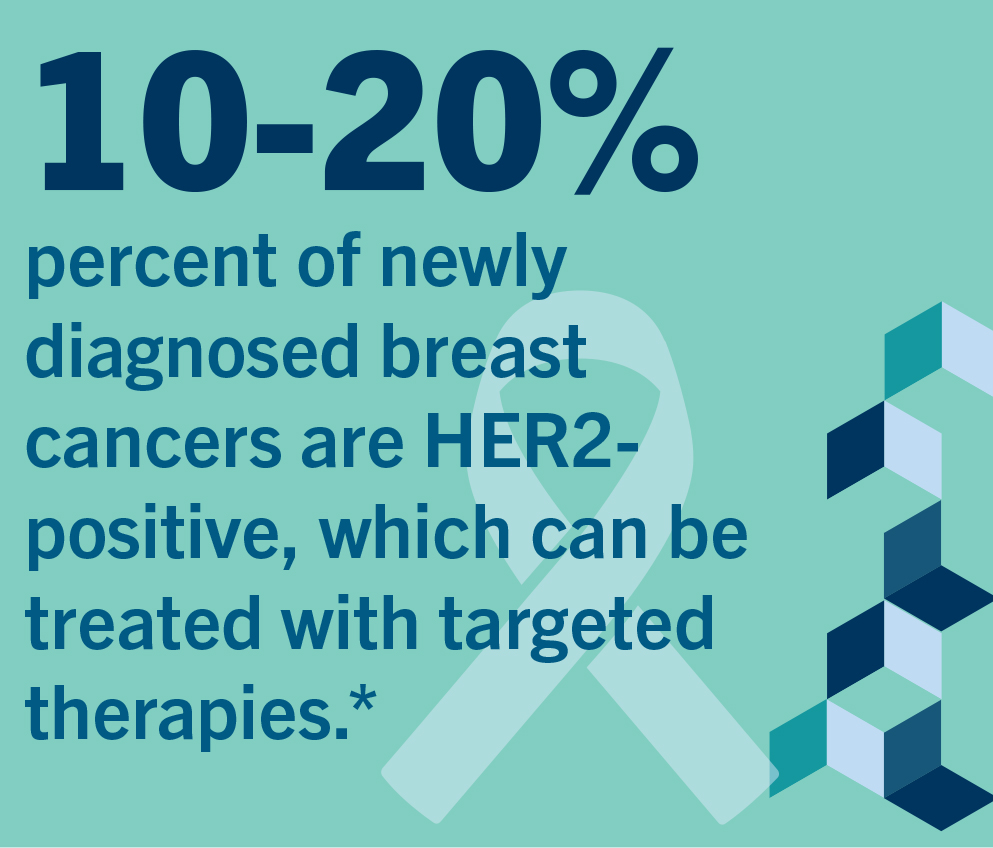 Rebecca was 29 years old when she found a lump in her breast and was diagnosed with Stage 2 breast cancer. Given her family’s extensive history of cancer, including an aunt and grandmother who died from breast cancer, she thought her diagnosis was a death sentence.
Rebecca was 29 years old when she found a lump in her breast and was diagnosed with Stage 2 breast cancer. Given her family’s extensive history of cancer, including an aunt and grandmother who died from breast cancer, she thought her diagnosis was a death sentence.
What Rebecca didn’t know was that her care team had sent her tissue samples out for biomarker testing, a step that would ultimately save her life. A month after her initial diagnosis, Rebecca learned that she had HER2-positive breast cancer, a cancer subtype that is susceptible to very effective targeted treatments. Rebecca was treated with six cycles of a targeted chemotherapy before having a double mastectomy. She was then prescribed a year of immunotherapy, a treatment that uses a person’s own immune system to fight cancer. Rebecca’s insurance, which she receives through her husband’s employer, would only pay for six months of immunotherapy, which meant she had to find financial assistance in the form of copay cards to help pay for her treatment.
Five years after her diagnosis, Rebecca has no signs of disease, was able to complete a master’s in public health, and is currently pursuing her EdD in Education. It’s a far different outcome from what might have been had she not had biomarker testing. Without the information that the biomarker testing provided, Rebecca’s treatment would have included “one-size-fits-all” chemotherapy with uncertain effectiveness for stopping the growth of her cancer and significant negative side effects that would have impacted her quality of life.
Rebecca was lucky. She benefited from the advances in biomarker testing to pinpoint mutations in the cancer, and enabled her clinicians to deploy treatments specific to those mutations. But many insurance plans still limit the use of biomarker testing, leaving patients and doctors without key information to decide which treatments will work best.
*Susan G. Komen Foundation, https://www.komen.org/breast-cancer/metastatic/metastatic/her2-targeted-therapies-for-metastatic-breast-cancer/
Guidelines for Coverage
By relying on the objective review of available data, clinicians, patients, and insurers can make the best decisions about coverage and treatment choices. Using evidence-based clinical treatment guidelines ensures that testing and treatment decisions reflect the latest knowledge of a cancer and its biomarkers.
Several highly regarded professional associations, including the National Comprehensive Cancer Network (NCCN), the American Association of Clinical Oncology (ASCO), and the College of American Pathologists (CAP) have developed biomarker testing and treatment guidelines. Insurers and employers should mandate the reference of these organizations in their policies, ensuring that what is recommended in their guidelines is covered and can be accessed in a timely way by covered persons with a cancer diagnosis.

Links in image: 13, 14, 15, 16 and pathologists.
In rectal cancer, a very difficult rare cancer, data from a small study released in 2022 found that using immunotherapy for patients with a specific biomarker prior to their surgery could be curative. Every patient in the study had a complete response to the therapy, preventing the need for more toxic treatments over time12.
A 2015 study comparing the impact of targeted therapy in various metastatic cancers found that patients who received targeted therapy had significantly improved outcomes when compared with those who were treated with traditional therapies9.
A particularly challenging aspect of many advanced stage tumors is their ability to change (or mutate), developing ways to resist initial therapies. For this reason, it is often important to conduct repeated biomarker testing throughout a patient’s continuum of care. This can help clinicians tailor the most effective treatment approaches34.
“Patients with certain mutations in their cancer can now be treated with ‘targeted therapies,’ which can be more effective than traditional chemotherapies. But if patients can’t get biomarker testing because of insurance coverage or other barriers, they may be denied the most appropriate and effective treatments available.”
--Len Lichtenfeld, MD, MACP
Chief Medical Officer, Jasper Health
Coverage and Reimbursement Gaps for Biomarker Testing29
 In 2018, the US Centers for Medicare and Medicaid Services (CMS) determined that Next Generation Sequencing (NGS) is a diagnostic laboratory test that is reasonable and necessary in specific circumstances30.
In 2018, the US Centers for Medicare and Medicaid Services (CMS) determined that Next Generation Sequencing (NGS) is a diagnostic laboratory test that is reasonable and necessary in specific circumstances30.
However, despite the potential for significantly improved outcomes with the use of biomarker-directed treatments, many cancer patients are still not receiving this testing. One reason is that, while private payers insure more than two thirds of the US population, many health insurance policies do not yet adequately cover guideline recommended tests. There are various reasons for these coverage gaps19 as payers use different approaches in making coverage decisions31. Evidence from an FDA approval is generally the most significant determinant of coverage. For LDTs, payers often look to professional guidelines and clinical trial data to determine whether the test result is clinically meaningful and useful.
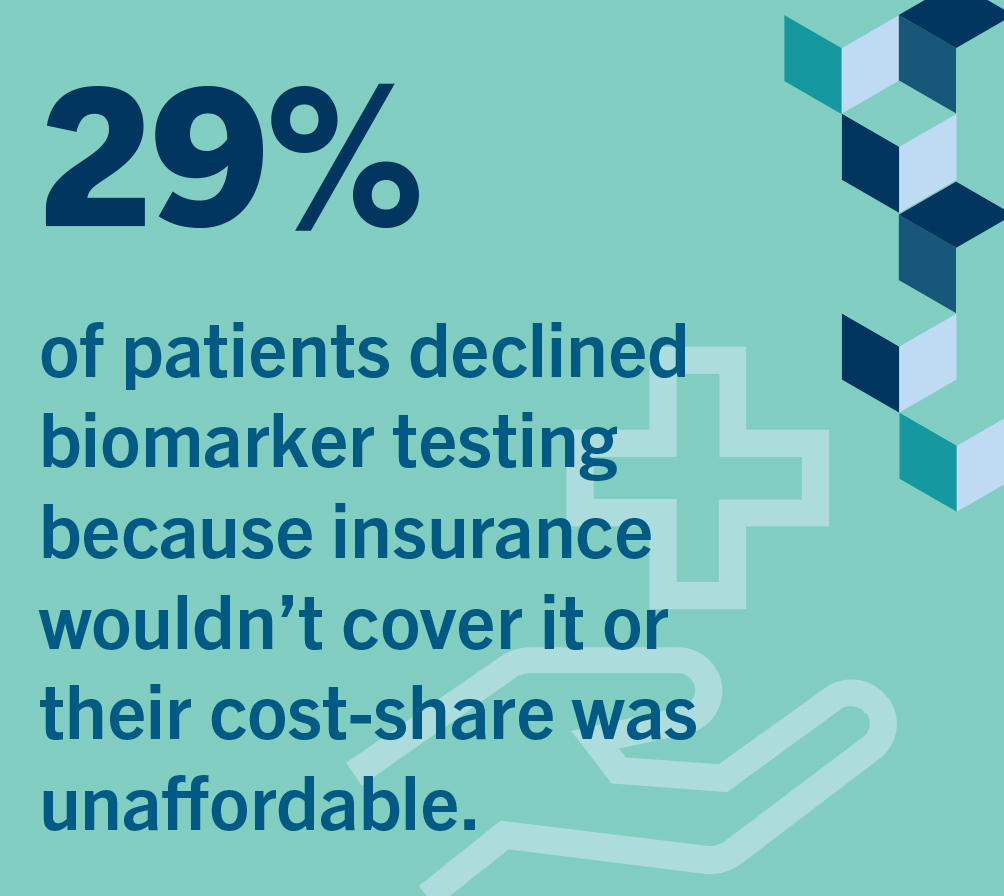
A patient survey completed in 2020 indicated that 29% of patients did not have testing done due to lack of insurance coverage or because they could not afford the out-of-pocket costs18. Lack of access to biomarker testing and coverage barriers are issues for many patients but appear to have a greater impact among racial and ethnic minorities and socioeconomically disadvantaged patients17. In the specific case of Non- Small Cell Lung Cancer (NSCLC), the most common type of lung cancer, despite the availability of extremely effective biomarker-directed therapies, up to one-fourth of these patients don’t receive biomarker testing to determine whether a specific available treatment might work for them.
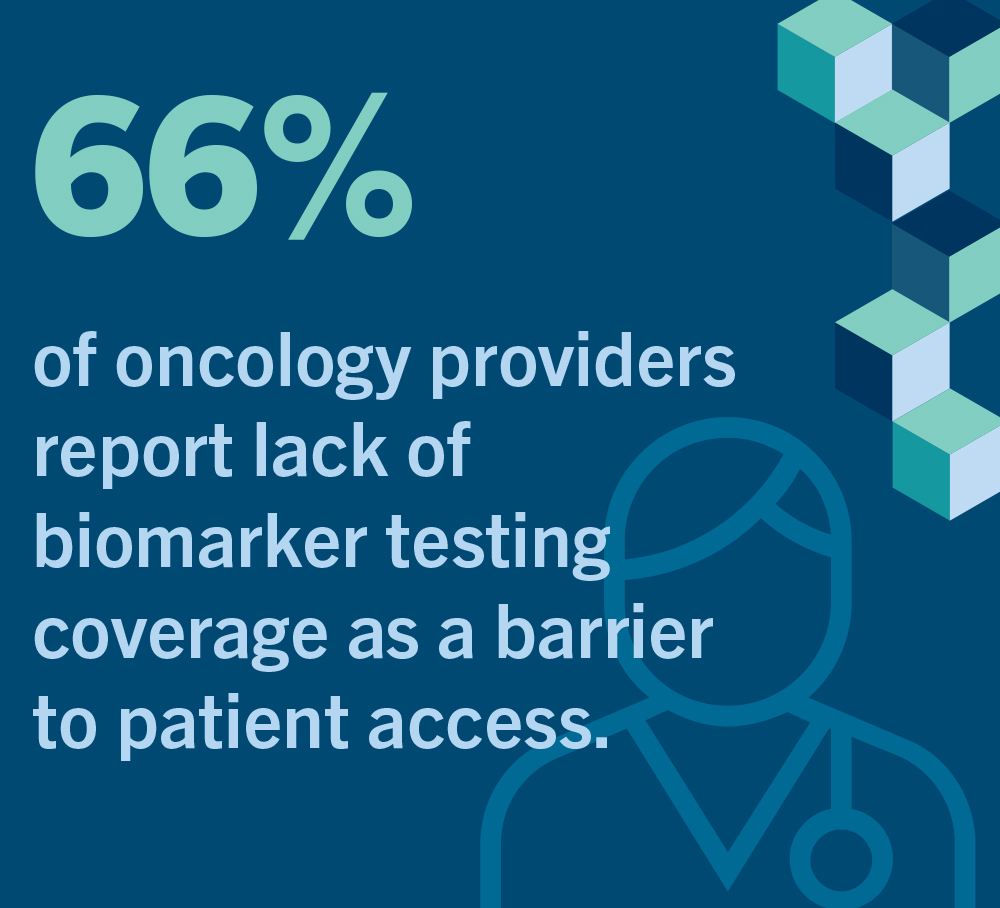 In a 2021 survey, two-thirds of oncologists reported that their patients face significant or moderate insurance barriers in accessing biomarker testing, and almost half of these healthcare providers reported needing to “often” or “always” seek prior authorization from insurers before they could proceed with appropriate testing for their patients7.
In a 2021 survey, two-thirds of oncologists reported that their patients face significant or moderate insurance barriers in accessing biomarker testing, and almost half of these healthcare providers reported needing to “often” or “always” seek prior authorization from insurers before they could proceed with appropriate testing for their patients7.
In general, even when insurance coverage exists for recommended biomarker testing, health plans often require significant out-of-pocket cost sharing and prior authorization, which can add hurdles and delays to a patient’s access to this needed care.
These barriers have not gone unnoticed. At the state level, as of January 2023, nine states have already passed laws requiring health plans to cover biomarker testing and limiting the use of prior authorization on biomarker testing; several other states are currently considering similar legislation with more expected to follow20.
Biomarker Testing Can Reduce Costs
Biomarker testing can ensure that patients receive therapies from which they are most likely to benefit while avoiding treatments that are unlikely to be effective or could cause harm22. In this way, biomarker testing can potentially limit costs to the healthcare system, insurers, and employers.
“In the age of precision oncology, pathologists need to be thinking about constantly. Biomarkers can be part of making the correct cancer diagnosis, deciding the best cancer treatment, or both!”
--Sarah E. Kerr, MD
Pathologist, Hospital Pathology Associates, Divisions of Cytopathology, Gynecologic and Molecular Pathology, Allina Health Laboratories, Allina Health Cancer Institute
There are multiple examples of this important benefit:
A 2020 study evaluating the use of biomarkers to direct targeted maintenance therapy for certain ovarian cancer patients found it could yield significant savings23.
A 2015 study found that treatment based on testing for key biomarkers in NSCLC patients is more cost effective than failing to use biomarker testing and treating these patients with chemotherapy24.
A 2012 study of metastatic colorectal cancer revealed that using an identified biomarker to direct treatment decisions can ensure that only those patients who may benefit from a treatment receive it, while avoiding unnecessary costs and harm to those unlikely to benefit. This study found that it is possible to increase expected overall survival while saving approximately $7,500 per patient when compared to using non-biomarker-directed treatment25.
It has been estimated that using specific biomarker testing could save more than $600 million by ensuring that only those metastatic colorectal cancer patients likely to benefit from a specific therapy would receive it26.
A 2007 study of early-stage breast cancer patients with certain characteristics found that using a validated biomarker to guide treatment decisions could lead to more than eight years of increased quality-adjusted survival while significantly reducing overall financial costs. In this study, biomarker-guided therapy was estimated to save over $1,000 per life year- spared when compared with traditional chemotherapy27.
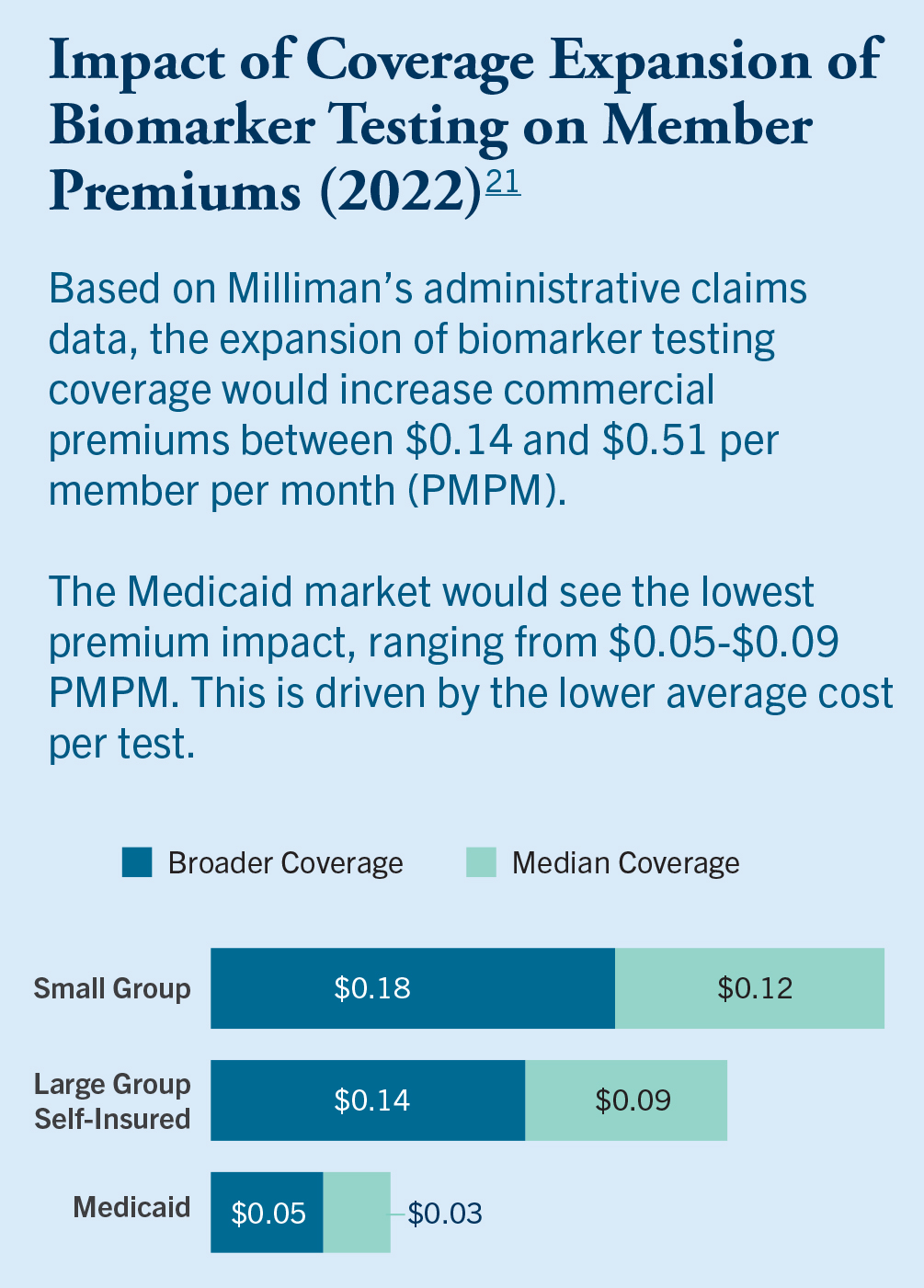 It has also been demonstrated that including guideline recommended biomarker testing in a benefit design is not expensive for payers. Studies of the potential cost for expanding coverage of biomarker testing among various types of insurance indicate an extremely small per member/per-month impact21.
It has also been demonstrated that including guideline recommended biomarker testing in a benefit design is not expensive for payers. Studies of the potential cost for expanding coverage of biomarker testing among various types of insurance indicate an extremely small per member/per-month impact21.
Of course, beyond the economic value offered by biomarker testing, the human impact is enormous. For patients with a treatable cancer mutation, timely biomarker testing can literally mean the difference between life and death.
“Biomarker testing makes a huge difference in how I treat patients diagnosed with lung cancer. Getting patients on the right treatment at the right time can extend their lives by years. Every patient with lung cancer should be able to access this important diagnostic tool.”
--Dr. Ryan Nguyen, DO
Lung cancer oncologist, Assistant Professor of Clinical Medicine, University of Illinois at Chicago
Case Study
 Nanci Carney
Nanci Carney
East Silver Lake, NH
Melanoma
Watch Nanci's story »
In April 2021, Nanci noticed an unusual mole under her arm while showering. She knew she’d not seen it before and as a longtime volunteer with the American Cancer Society, she knew she should get it checked out. She was able to get an appointment with a dermatologist relatively quickly and was surprised when the doctor told her he would remove the mole immediately and send it off for a biopsy.
Two weeks later, when her dermatologist called at 8:30 PM on a Friday night, she knew it wasn’t going to be good news. He asked if she knew what melanoma was, and Nanci’s cancer journey began.
Nanci had surgery to remove the mole and the area around it to be sure that all the cancer had been removed. The good news was that there was no sign of spread in the tissue samples. The traditional next step would be another surgery to remove lymph nodes in the surrounding area to ensure that the cancer hadn’t spread to her lymphatic system.
Nanci’s doctor was hesitant to do the second surgery because removing those lymph nodes very often leads to lymphedema, a serious long-term condition that causes swelling due to a build-up of lymph fluid in the body. Instead, he recommended biomarker testing, which would tell them more about Nanci’s particular cancer, the chances of it recurring, and help them determine whether additional surgery was the best course.
Nanci thought she had good health insurance through her employer, and her doctor had been told by the plan’s insurance representative that they covered biomarker testing. Tissue samples from Nanci’s surgery were sent to the lab, and she soon received the great news that her cancer was unlikely to recur, and she would not need to undergo the additional surgery with a high risk of life-long complications.
A month later, despite their having told her doctor that it was covered, Nanci’s insurance company denied coverage for the biomarker testing and said she was responsible for the entire $8,500 bill. They claimed the testing was not medically necessary. Even though the biomarker testing spared Nanci from a second surgery that would have cost far more than the test, her insurer stated, “there is no proof, or not enough proof, that this test improves health outcomes.”
In reality, however, biomarker testing not only improved Nanci’s health outcome by sparing her unnecessary surgery with a high risk of later complications, but it also saved the insurance company the cost of that surgery and any treatments that may have followed had she developed lymphedema.
Covering Biomarker Testing in Plan Design
CancerCare calls on employers to insist that health coverage for their employees provides access to approved and recommended biomarker testing at diagnosis, transition in treatment plan, cancer recurrence, and any time other testing shows significant changes in the cancer or its impact on the patient.
Recommendations**
Coverage should follow guideline recommendations and FDA-approved uses.
- Payers should provide coverage for multi-gene panel testing as indicated by expert guidelines, and in cases where its use is more efficient than single-gene tests, a single gene test does not exist, or when tissue availability is too limited for use of multiple single gene tests.
- Payers should cover as wide a range of tests as possible, including biomarker tests, next generation sequencing (NGS), companion diagnostics, and lab-developed tests as indicated by guidelines and FDA approvals.
- Coverage should be extended to testing that can be used to inform clinicians and patients of potential clinical trial opportunities.
- Coverage should be updated as new tests are approved by the FDA or added to expert guidelines. There should not be a waiting period before coverage takes effect.
Coverage should ensure access to biomarker testing at time of diagnosis and/or recurrence.
- Coverage of biomarker testing should not be restricted to a single occurrence. It should allow for additional testing to identify a change in the genetic makeup of the patient’s cancer or to monitor disease progression.
Coverage should be comprehensive.
- Payers should provide coverage and access to appropriate services for expert interpretation of biomarker tests, including from out-of-network experts, with no or minimal cost-sharing.
- Coverage should extend to genetic testing for inherited risk (and familial links) and genetic counseling.
- Coverage should include psychosocial counseling as needed.
- Coverage of biomarker testing should not be limited to specific cancer types or stages.
- Consider offering navigation and psychosocial support to covered employees and other beneficiaries with cancer to help secure coverage for biomarker testing and related services.
Barriers to coverage should be minimized.
- Coverage should avoid barriers to physician ordering and patients undergoing timely biomarker testing such as prior authorization, referral restrictions, and denials that are likely to be overturned on appeal.
- Payers should avoid burdensome cost-sharing that could impede equitable access to biomarker tests. Any cost-sharing should be via a flat co-payment, rather than percentage-based co-insurance.
**Based on principles from the American Cancer Society Cancer Action Network (ACS CAN) model state legislation28
Questions to Ask Your Benefit Consultants and Insurance Providers
Coverage Tied to Guidelines
Questions to ask:
- Does the plan include coverage for all expert guideline recommended cancer biomarker testing? (NCCN, ASCO, CAP)
- Does the plan have a waiting period before newly FDA-approved biomarker tests are covered?
- Is the plan regularly updated as new guidelines and FDA-approved biomarker tests are available?
- If so, does this happen on a real-time basis or periodically?
- If periodically, how often?
Covered Tests
Questions to ask:
- Does the plan include coverage for all expert guideline recommended cancer biomarker testing? (NCCN, ASCO, CAP)
- Does the plan include coverage for all FDA-approved biomarker tests?
- Does the plan cover next generation sequencing (NGS) biomarker tests?
- Does the plan cover lab-developed tests (LDTs)?
- Does the plan cover companion diagnostic tests?
- Does the plan cover biomarker testing to determine a patient’s potential clinical trial eligibility?
- Does the plan specify in-network test providers or specific companies who make tests in its biomarker testing coverage?
- What (if any) limitations does the plan place on types or providers of covered tests?
- If so, detail those limitations and their rationale.
- Does the plan allow for testing from appropriate labs (even if out-of-network)?
- If so, what are the associated out-of-pocket costs to patients?
Test Timing and Utilization Review
Questions to ask:
- Does the plan cover biomarker testing at the time of cancer diagnosis?
- Does the plan cover biomarker testing at the time of cancer recurrence?
- Does the plan cover biomarker testing during transitions in treatment plans, and when other testing shows significant changes in the cancer or its impact on the patient?
- Does the plan have any limitations or restrictions on the timing of biomarker tests covered?
- If so, explain the limitations and provide their rationale including whether the restrictions are consistent with current expert guidance from NCCN, ASCO, and CAP.
- Does the plan require prior authorization or limitations on referral for biomarker testing?
- If so, please provide details on time limits for decisions and requests for additional information, and actions if the time limits are not met.
- What is the rate of approval and timing on appeals when biomarker testing is denied? (if appeals are regularly approved, why are denials being made to begin with?)
Coverage Limitations
Questions to ask:
- Does the plan restrict biomarker testing coverage based on type or stage of cancer?
- If so, explain the restrictions and provide their rationale including whether the restrictions are consistent with current expert guidance from NCCN, ASCO, and CAP.
- Does the plan include restrictions on the number of times a patient can receive biomarker testing?
- If so, explain the restrictions and provide their rationale including whether the restrictions are consistent with current expert guidance from NCCN, ASCO, and CAP.
- Does the plan include prior authorization requirements or restrictions on referral for biomarker testing services (including biopsy procedures, blood draws, etc.)?
- If so, explain the requirements/restrictions and provide their rationale including whether they are consistent with current expert guidance from NCCN, ASCO, and CAP.
- What is the cost-sharing requirement for biomarker testing services?
Interpreting Results
Questions to ask:
- Does the plan cover pathology and physician billing for interpretation of biomarker results?
- Does the plan allow patients to seek additional expert opinions about their results, including from out-of- network experts?
- If so, what are the cost-sharing requirements?
Coverage of Ancillary Services
Questions to ask:
- Does the plan cover genetic testing for inherited risk?
- If so, please provide details on coverage, prior authorization requirements, and patient cost-sharing requirements.
- Does the plan cover genetic counseling and related psychosocial services for patients?
- If so, please provide details on coverage, prior authorization requirements, and patient cost-sharing requirements.
Videos
Glossary of Terms
Analytical Validity, Clinical Validity, Clinical Utility
Analytical validity describes how accurately and reliably the test detects and measures a biomarker of interest. Clinical validity provides some insight into how well the test relates to the clinical outcome of interest, e.g., response to therapy, survival, etc. Clinical utility describes whether the results of the test provide information that can contribute to and improve current optimal management of the patient’s disease.33
Biomarker
Biomarkers are defined characteristics in the body that are measured as indicators of health, disease, or a response to an exposure or intervention, including treatment. Biomarkers can help diagnose a disease, or predict future disease severity or outcomes, like measurements of blood pressure as an indicator of cardiovascular risk or measurements of blood sugar in diabetes. Biomarkers also are used to identify the best treatment for a patient, to monitor the safety of a therapy, or to find out if a treatment is having the desired effect on the body.5
Biomarker Testing
Biomarker testing is a way to look for genes, proteins, and other substances (called biomarkers or tumor markers) that can provide information about a cancer. Each person’s cancer has a unique pattern of biomarkers. Some biomarkers affect how certain cancer treatments work.34
Clinical Laboratory Improvement Amendments (CLIA)
The Clinical Laboratory Improvement Amendments of 1988 (CLIA) regulations include federal standards applicable to all US facilities or sites that test human specimens for health assessment or to diagnose, prevent, or treat disease.35
Companion Diagnostic
A companion diagnostic test provides information that is essential for the safe and effective use of a corresponding drug or biological product. The test helps a health care professional determine whether a particular therapeutic product’s benefits to patients will outweigh any potential serious side effects or risks. Companion diagnostics can identify patients who are most likely to benefit from a particular therapeutic product, identify patients likely to be at increased risk for serious side effects due to treatment with a particular therapeutic product, or monitor response to treatment with a particular therapeutic product for the purpose of adjusting treatment to achieve improved safety or effectiveness.36
DNA
DNA is the molecule that carries genetic information for the development and functioning of an organism. DNA is self-replicating material that is present in nearly all living organisms as the main constituent of chromosomes. It is the carrier of genetic information. The DNA from any two people is 99.9% identical. The differing 0.1% contains variations that influence individuals’ uniqueness.37
Genes
Genes are the basic unit of heredity passed from parent to child. Genes are made up of sequences of DNA and are arranged, one after another, at specific locations on chromosomes in the nucleus of cells. In humans, genes vary in size from a few hundred DNA bases to more than 2 million bases.38
Gene Mutations
A gene mutation is a change in one or more genes. Some mutations can lead to genetic disorders or illnesses.39
Genetic Testing for Inherited Risk
Genetic testing looks for specific inherited changes (variants) in a person’s genes. Genetic variants can have harmful, beneficial, neutral (no effect), or unknown or uncertain effects on the risk of developing diseases. Harmful variants in some genes are known to be associated with an increased risk of developing cancer. These inherited variants are thought to contribute to about 5-10% of all cancers.51
Genome
The genome is the complete set of DNA (genetic material) in an organism. In people, almost every cell in the body contains a complete copy of the genome. The genome contains all the information needed for a person to develop and grow.52
Genome Sequencing
Genome sequencing is figuring out the order of DNA nucleotides, or bases, in a genome—the order of As, Cs, Gs, and Ts that make up an organism’s DNA. The human genome is made up of over 3 billion of these genetic letters.53
Immunotherapy
Immunotherapy is treatment that uses a person’s own immune system to fight cancer. Immunotherapy can boost or change how the immune system works so it can find and attack cancer cells.40
Lab Developed Test (LDT)
A laboratory developed test (LDT) is a type of diagnostic test that is designed, manufactured, and used within a single laboratory. LDTs can be used to measure or detect a wide variety of analytes (substances such as proteins, chemical compounds like glucose or cholesterol, or DNA), in a sample taken from a human body. Some LDTs are relatively simple tests that measure single analytes, such as a test that measures the level of sodium. Other LDTs are complex and may measure or detect one or more analytes. For example, some tests can detect many DNA variations from a single blood sample, which can be used to help diagnose a genetic disease.41
Liquid Biopsy
Liquid biopsy is a test done on a sample of blood to look for cancer cells from a tumor that are circulating in the blood or for pieces of DNA from tumor cells that are in the blood. A liquid biopsy may be used to help find cancer at an early stage.42
Molecular Profiling
Molecular profiling is a laboratory method that uses a sample of tissue, blood, or other body fluid to look for certain genes, proteins, or other molecules that may be a sign of a disease or condition, such as cancer.43
Molecular Subtype
The molecular subtype of a cancer is based on the genes the cancer cells express, which control how the cells behave.44
Next Generation Sequencing
Next-generation sequencing (NGS) is a massive parallel sequencing technology that offers ultra-high throughput, scalability, and speed. There are multiple different NGS platforms that use different sequencing technologies. All NGS platforms perform sequencing of millions of small fragments of DNA in parallel. Bioinformatics analyses are used to piece together these fragments by mapping the individual reads to the human reference genome. Each of the three billion bases in the human genome is sequenced multiple times, providing high depth to deliver accurate data and insight into unexpected DNA variation.45
Panel Test
A panel test is a laboratory procedure in which a series of tests is performed on one specimen.46
Pathologist
A pathologist is a medical healthcare provider who examines bodies and body tissues. They are also responsible for performing lab tests. A pathologist helps other healthcare providers determine diagnoses and is an important member of the treatment team.47
Precision Medicine
Precision medicine looks at the genetics, environment, and lifestyle of a person to select treatment that could work best for them.48
Prior Authorization
Prior authorization (PA) refers to a requirement by health plans for patients to obtain approval of a health care service or medication before the care is provided. It is supposed to contain costs, ensure that a treatment is medically necessary, and protect patient safety. PA requirements can delay treatment, restrict access to medications or specialists, and increase costs for patients and clinical practices.49
Single Analyte Test
A single analyte test is an assay designed for testing or measuring only a single analyte, which is a chemical substance in a fluid or other specimen from the body.50
Targeted Therapy
A targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific types of cancer cells with less harm to normal cells. Some targeted therapies block the action of certain enzymes, proteins, or other molecules involved in the growth and spread of cancer cells.54
Works Cited
Figure 1: Zhong, L., Li, Y., Xiong, L., Wang, W., Wu, M., Yuan, T., Yang, W., Tian, C., Miao, Z., Wang, T., & Yang, S. (2021, May 31). Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Nature News. Retrieved December 13, 2022, from
https://www.nature.com/articles/s41392-021-00572-w
Cancer genomics. Genome.gov. Retrieved October 21, 2022, from https://www.genome.gov/dna-day/15-ways/cancer-genomics
Vidwans, S. J., Turski, M. L., Janku, F., Garrido-Laguna, I., Munoz, J., Schwab, R., Subbiah, V., Rodon, J., Kurzrock, R. (2014). A framework for genomic biomarker actionability and its use in clinical decision making. Oncoscience, 1(10), 614–623. https://doi.org/10.18632/oncoscience.90
A white paper on the need for consistent terms for testing in precision medicine. Retrieved October 21, 2022, from https://media.cancercare.org/publications/original/409-Consistent_Testing_Terminology_Whitepaper_Final_070720.pdf
Biomarker testing for cancer treatment. National Cancer Institute. Retrieved October 21, 2022, from https://www.cancer.gov/about-cancer/treatment/types/biomarker-testing-cancer-treatment
Center for Drug Evaluation and Research. About biomarkers and qualification. US Food and Drug Administration. Retrieved October 21, 2022, from https://www.fda.gov/drugs/biomarker-qualification-program/about-biomarkers-and-qualification
Mattes, W. B., Goodsaid, F. (2017). Regulatory landscapes for biomarkers and diagnostic tests: Qualification, approval, and role in clinical practice. Experimental Biology and Medicine, 243(3), 256–261. https://doi.org/10.1177/1535370217739629
Survey Findings Summary: Understanding Provider Utilization of Cancer Biomarker Testing Across Cancers, American Cancer Society, Cancer Action Network, December 2021, https://www.fightcancer.org/sites/default/files/national_documents/provider_utilization_of_biomarker_testing_polling_memo_dec_2021.pdf
Improving Access to Biomarker Testing - American Cancer Society Cancer Action Network. Retrieved October 21, 2022, from https://www.fightcancer.org/sites/default/files/Improving%20Access%20to%20Biomarker%20Testing_FINAL.pdf
Schwaederle, M., Zhao, M., Lee, J. J., Eggermont, A. M., Schilsky, R. L., Mendelsohn, J., Lazar, V., Kurzrock, R. (2015). Impact of precision medicine in diverse cancers: A meta-analysis of Phase II clinical trials. Journal of Clinical Oncology, 33(32), 3817–3825. https://doi.org/10.1200/jco.2015.61.5997
Musika, W., Kamsa-Ard, S., Jirapornkul, C., Santong, C., Phunmanee, A. (2021). Lung cancer survival with current therapies and new targeted treatments: A comprehensive update from the SRINAGARIND Hospital-Based Cancer Registry from (2013 to 2017). Asian Pacific Journal of Cancer Prevention, 22(8), 2501–2507. https://doi.org/10.31557/apjcp.2021.22.8.2501
Sicklick, J. K., Kato, S., Okamura, R., Patel, H., Nikanjam, M., Fanta, P. T., Hahn, M. E., De, P., Williams, C., Guido, J., Solomon, B. M., McKay, R. R., Krie, A., Boles, S. G., Ross, J. S., Lee, J. J., Leyland-Jones, B., Lippman, S. M., Kurzrock, R. (2021). Molecular profiling of advanced malignancies guides first-line N-of-1 treatments in the I-predict treatment-naïve study. Genome Medicine, 13(1). https://doi.org/10.1186/s13073-021-00969-w
Cercek, A., Lumish, M. A., Sinopoli, J. C., Weiss, J. A., Shia, J., Stadler, Z. K., Yaeger, R., Smith, J. J., Saltz, L. B., El Dika, I. H., Crane, C. H., Romesser, P. B., Iyer, K., Paty, P., Garcia-Aguilar, J., Gonen, M., Gollub, M. J., Weiser, M. R., Schalper, K. A., Diaz, L. A. (2022). Single agent PD-1 blockade as curative-intent treatment in mismatch repair deficient locally advanced rectal cancer. Journal of Clinical Oncology, 40(17_suppl). https://doi.org/10.1200/jco.2022.40.17_suppl.lba5
Treatment by cancer type. NCCN. Retrieved October 21, 2022, from https://www.nccn.org/guidelines/category_1
Biomarkers compendium. NCCN. Retrieved October 21, 2022, from https://www.nccn.org/compendia-templates/compendia/biomarkers-compendium
Guidelines, tools, and resources. ASCO. Retrieved October 21, 2022, from https://old-prod.asco.org/practice-patients/guidelines
Current CAP guidelines. College of American Pathologists. Retrieved October 21, 2022, from https://www.cap.org/protocols-and-guidelines/current-cap-guidelines
Bruno, D. S., Hess, L. M., Li, X., Su, E. W., Zhu, Y. E., & Patel, M. (2021). Racial disparities in biomarker testing and clinical trial enrollment in non-small cell lung cancer (NSCLC). Journal of Clinical Oncology, 39(15_suppl), 9005–9005. https://doi.org/10.1200/jco.2021.39.15_suppl.9005
Survivor Views: Biomarker Testing Survey Findings Summary. American Cancer Society Cancer Action Network (2020, September). Retrieved January 12, 2023, from https://www.fightcancer.org/sites/default/files/Survivor%20Views%20Biomarker%20Testing%20Polling%20Memo.pdf
Payer coverage policies of tumor biomarker testing. (2020, September). Retrieved October 21, 2022, from https://www.fightcancer.org/sites/default/files/ACS%20CAN%20and%20LUNGevity_Payer%20Coverage%20Policies%20of%20Tumor%20Biomarker%20Testing.pdf
Biomarker testing: Enacted laws. Retrieved October 21, 2022 from https://aimedalliance.org/biomarker-testing-enacted-laws/
The landscape of biomarker testing coverage in the United States. Milliman. (2022, February 15). Retrieved October 21, 2022, from https://www.milliman.com/en/insight/the-landscape-of-biomarker-testing-coverage-in-the-us
Committee on policy issues in the clinical development and use of biomarkers for molecularly targeted therapies: Keys to unlocking precision medicine. Retrieved October 21, 2022, from https://www.ncbi.nlm.nih.gov/books/NBK379341/
Gonzalez, R., Havrilesky, L. J., Myers, E. R., Secord, A. A., Dottino, J. A., Berchuck, A., Moss, H. A. (2020). Cost-effectiveness analysis comparing “PARP inhibitors-for-all” to the biomarker-directed use of PARP inhibitor maintenance therapy for newly diagnosed advanced stage ovarian cancer. Gynecologic Oncology, 159(2), 483–490. https://doi.org/10.1016/j.ygyno.2020.08.003
Romanus, D., Cardarella, S., Cutler, D., Landrum, M. B., Lindeman, N. I., Gazelle, G. S. (2015). Cost-effectiveness of multiplexed predictive biomarker screening in non-small-cell lung cancer. Journal of Thoracic Oncology, 10(4), 586–594. https://doi.org/10.1097/jto.0000000000000474
Behl, A. S., Goddard, K. A., Flottemesch, T. J., Veenstra, D., Meenan, R. T., Lin, J. S., Maciosek, M. V. (2012). Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer. JNCI: Journal of the National Cancer Institute, 104(23), 1785–1795. https://doi.org/10.1093/jnci/djs433
Mancl, E. E., Kolesar, J. M., Vermeulen, L. C. (2009). Clinical and economic value of screening for KRAS mutations as predictors of response to epidermal growth factor receptor inhibitors. American Journal of Health-System Pharmacy, 66(23), 2105–2112. https://doi.org/10.2146/ajhp090036
Lyman, G. H., Cosler, L. E., Kuderer, N. M., Hornberger, J. (2007). Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer. Cancer, 109(6), 1011–1018. https://doi.org/10.1002/cncr.22506
Recommendations to Improve Access to Biomarker Testing in Cancer, ACS CAN, September 2020, https://www.fightcancer.org/sites/default/files/Recommendations%20to%20Improve%20Access%20to%20Biomarker%20Testing.pdf
Trosman, J.R., Weldon C.B., Gradishar W.J., et al. (2018) From the Past to the Present: Insurer Coverage Frameworks for Next-Generation Tumor Sequencing. Value Health, 21(9):1062-1068. doi:10.1016/j.jval.2018.06.011. Accessed January 21, 2023. https://pubmed.ncbi.nlm.nih.gov/30224110/
Next generation sequencing (NGS) for Medicare beneficiaries with advanced cancer. CMS.gov Centers for Medicare & Medicaid Services. Retrieved October 23, 2022, from https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=296&CoverageSelection=National&KeyWord=NGS&KeyWordLookUp=Title&KeyWordSearchType=And&bc=gAAAACAACAAA
Trosman, J. R., Van Bebber, S. L., & Phillips, K. A. (2010). Coverage policy development for personalized medicine: private payer perspectives on developing policy for the 21-gene assay. Journal of oncology practice, 6(5), 238–242. https://doi.org/10.1200/JOP.000075
Sadigh, G., Goeckner, H. G., Kazerooni, E. A., Johnson, B. E., Smith, R. A., Adams, D. V., & Carlos, R. C. (2022). State legislative trends related to biomarker testing. Cancer, 128(15), 2865–2870. https://doi.org/10.1002/cncr.34271
Grosse S.D., Kalman L., Khoury M.J. (2010). Evaluation of the validity and utility of genetic testing for rare diseases. Adv Exp Med Biol. 686:115-31. doi: 10.1007/978-90-481-9485-8_8. PMID: 20824443. Accessed January 21, 2023. https://pubmed.ncbi.nlm.nih.gov/20824443/
Biomarker testing for cancer treatment. National Cancer Institute. Retrieved October 23, 2022, from https://www.cancer.gov/about-cancer/treatment/types/biomarker-testing-cancer-treatment#will-biomarkernbsptesting-forcancer-treatment-help-me
Clinical Laboratory Improvement amendments (CLIA). Center for Devices and Radiological Health. US Food and Drug Administration. Retrieved October 23, 2022, from https://www.fda.gov/medical-devices/ivd-regulatory-assistance/clinical-laboratory-improvement-amendments-clia
Companion diagnostics. Center for Devices and Radiological Health. US Food and Drug Administration. Retrieved October 23, 2022, from https://www.fda.gov/medical-devices/in-vitro-diagnostics/companion-diagnostics
What is DNA? US National Library of Medicine. MedlinePlus Genetics. MedlinePlus. Retrieved October 23, 2022, from https://medlineplus.gov/genetics/understanding/basics/dna/
NCI dictionary of genetics terms. National Cancer Institute. Retrieved October 23, 2022, from https://www.cancer.gov/publications/dictionaries/genetics-dictionary/def/gene
American Cancer Society, Genes and Cancer, https://www.cancer.org/healthy/cancer-causes/genetics/genes-and-cancer/gene-changes.html
Immunotherapy for cancer. National Cancer Institute. Retrieved October 23, 2022, from https://www.cancer.gov/about-cancer/treatment/types/immunotherapy
Laboratory developed tests. Center for Devices and Radiological Health. US Food and Drug Administration. Retrieved October 23, 2022, from https://www.fda.gov/medical-devices/in-vitro-diagnostics/laboratory-developed-tests
NCI dictionary of cancer terms. National Cancer Institute. Retrieved October 23, 2022, from https://www.cancer.gov/publications/dictionaries/cancer-terms/def/liquid-biopsy
NCI dictionary of cancer terms. National Cancer Institute. Retrieved October 23, 2022, from https://www.cancer.gov/publications/dictionaries/cancer-terms/def/molecular-profiling
Zhao, L. Lee, V.H.F., Ng, M.N. Yan, H., Bijlsma, M.F. Molecular subtyping of cancer: Current status and moving toward clinical applications, Briefings in Bioinformatics, Volume 20, Issue 2, March 2019, Pages 572–584, https://doi.org/10.1093/bib/bby026
Behjati, S., & Tarpey, P. S. (2013). What is next generation sequencing?. Archives of disease in childhood. Education and practice edition, 98(6), 236–238. https://doi.org/10.1136/archdischild-2013-304340
What are the different types of genetic tests? US National Library of Medicine. MedlinePlus Genetics. MedlinePlus. Retrieved October 23, 2022, from https://medlineplus.gov/genetics/understanding/testing/types/
The pathologist. The Pathologist. Johns Hopkins Medicine. (2019). Retrieved October 23, 2022, from https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/the-pathologist
NCI dictionary of cancer terms. National Cancer Institute. Retrieved October 23, 2022, from https://www.cancer.gov/publications/dictionaries/cancer-terms/def/precision-medicine
Examining prior authorization in health insurance. Kaiser Family Foundation. (2022) Retrieved October 23, 2022, from https://www.kff.org/policy-watch/examining-prior-authorization-in-health-insurance/#:~:text=Prior%20authorization%20(also%20called%20%E2%80%9Cpreauthorization,medically%20necessary%20and%20otherwise%20covered
What are in vitro diagnostic tests and how are they regulated? Retrieved October23, 2022, from https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2019/05/what-are-in-vitro-diagnostic-tests-and-how-are-they-regulated
American Cancer Society. Retrieved October 23, 2022, from https://www.cancer.org/healthy/cancer-causes/genetics/genetic-testing-for-cancer-risk/understanding-genetic-testing-for-cancer.html
NIH, National Human Genome Research Institute, https://www.genome.gov/genetics-glossary/Genome
National Cancer Institute, NCI Dictionary of Cancer Terms. Retrieved January 20, 2023 from https://www.cancer.gov/publications/dictionaries/cancer-terms/def/genomic-sequencing
National Cancer Institute, Types of Cancer Treatment, Targeted Therapy to Treat Cancer. Retrieved January 20, 2023 from https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies
Acknowledgements
The Employers’ Prescription for Employee Protection: Biomarker Toolkit would not have been possible without the support and input of many organizations and individuals.
CancerCare Project Directors:
Carole Florman, MPA
Ellen Miller-Sonet, MBA, JD
Thank you for sharing your stories: Juanita Segura, Rebecca Muñoz, Nanci Carney
Thank you for sharing your resources: LUNGevity, American Cancer Society Cancer Action Network
Thank you for sharing your talent: Wendy Selig, Catherine Richards
The project was made possible with the generous support of Amgen, AstraZeneca, Blueprint Medicines, Pfizer, Sanofi, and Takeda.
©2023 CancerCare
